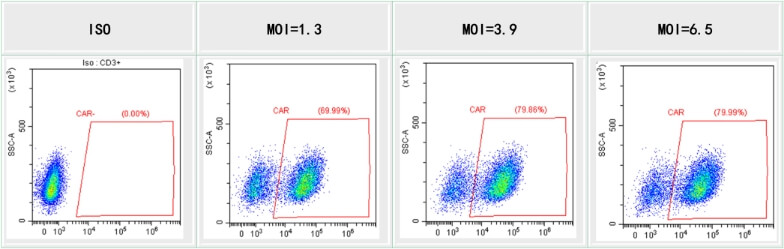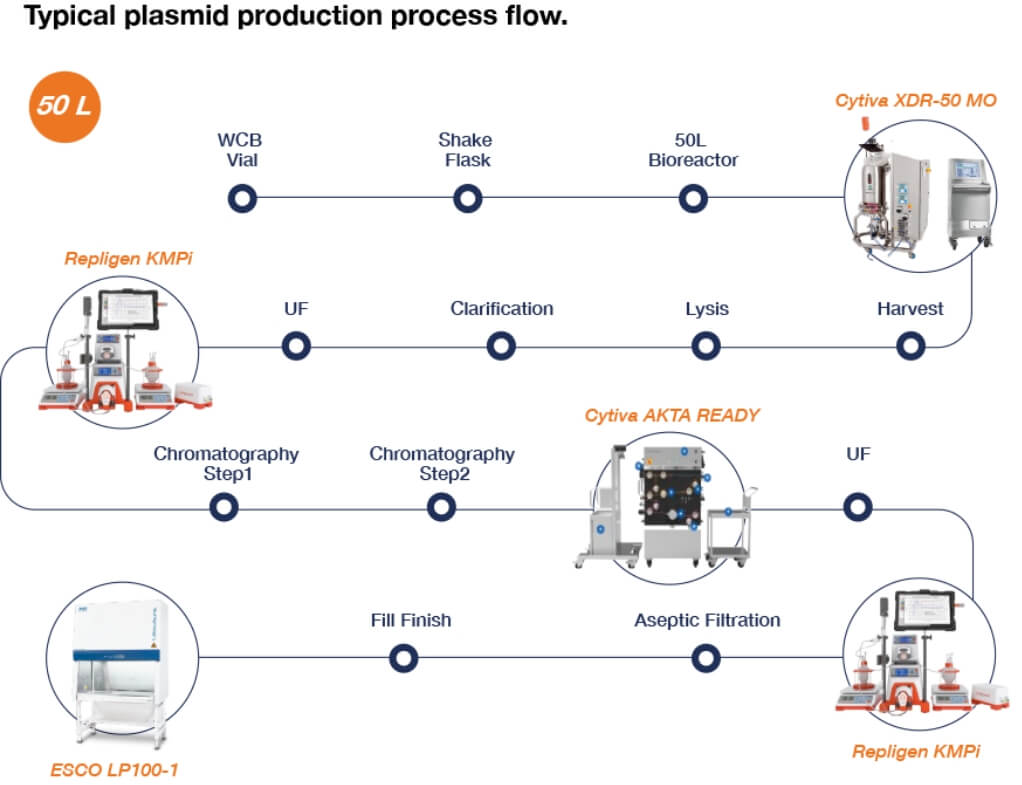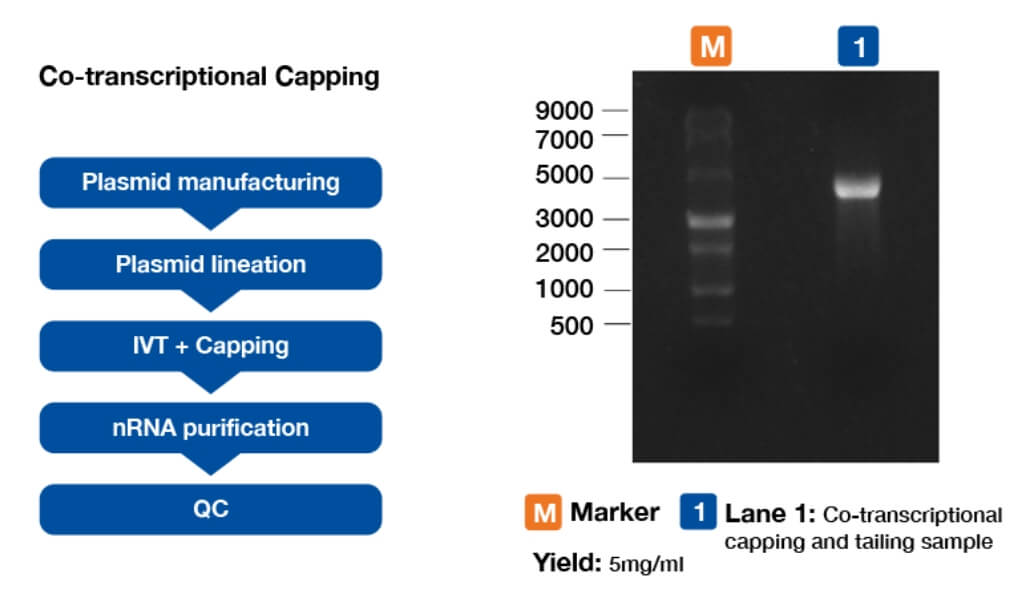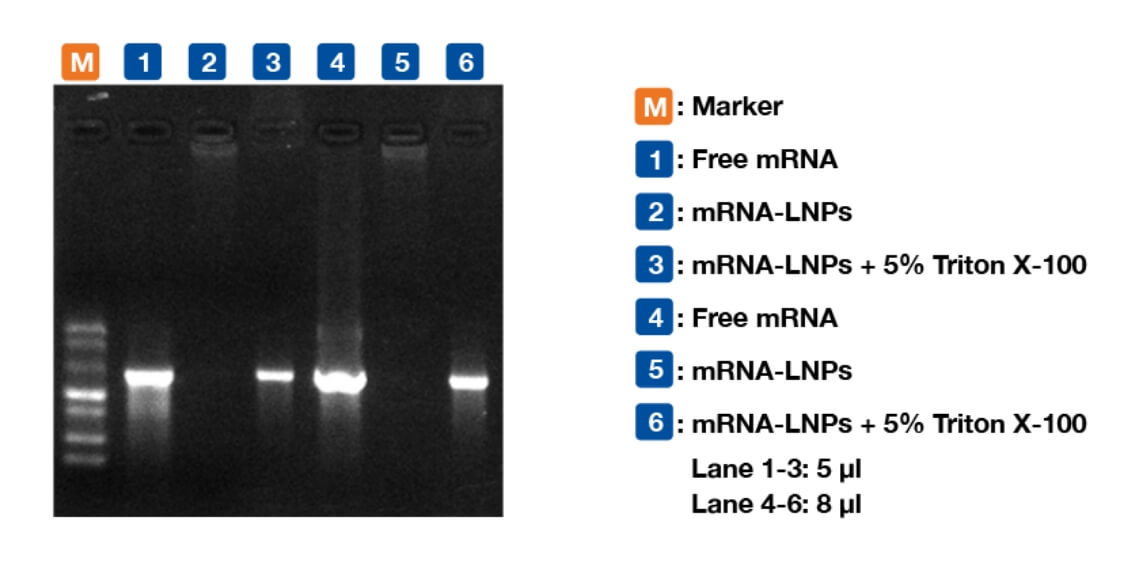
innovation centre
Latest new and upcoming events
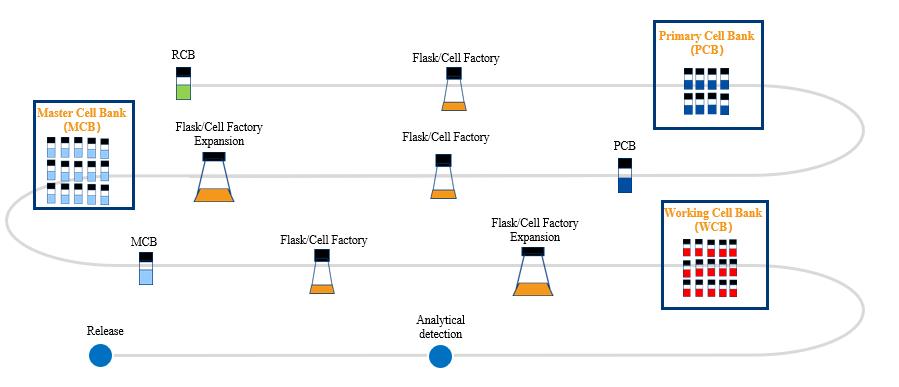
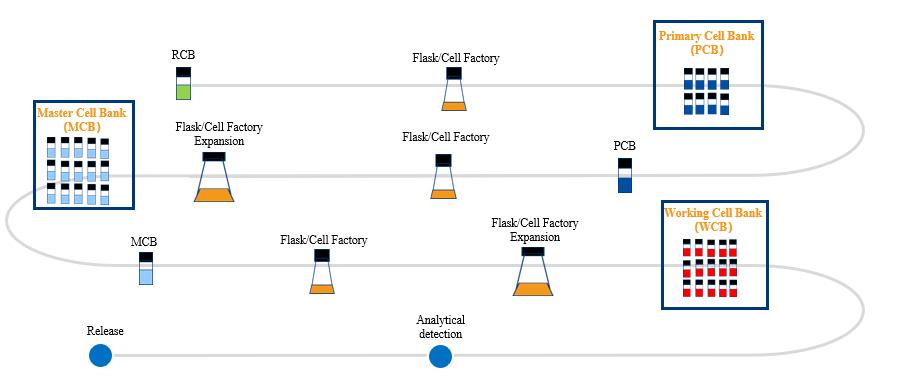
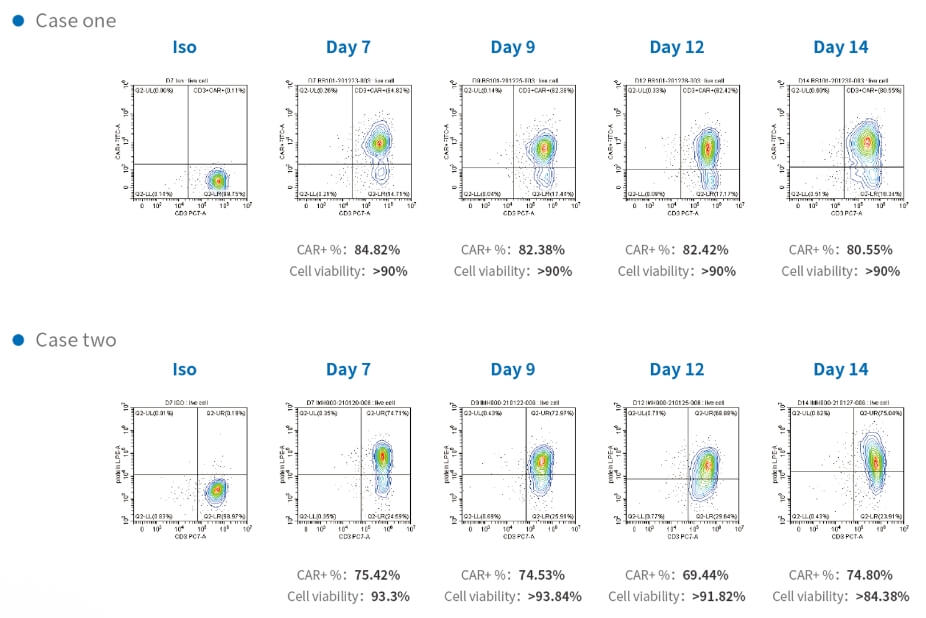
At Porton Advanced Solutions, we are continuously innovating with the aim to accelerate delivery of life-changing advanced therapies to patients.
Stay up-to-date with our latest news, publications and resources, and our upcoming events here.
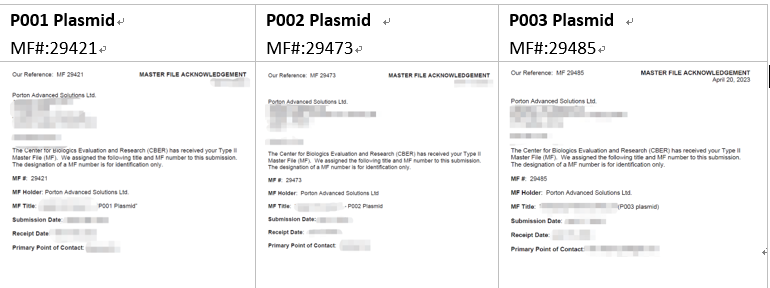
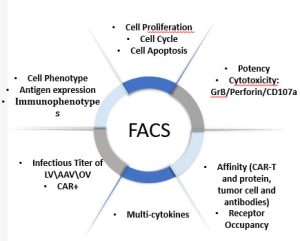
Porton Advanced Literature
Click to see more…
Downloads
Company
Brochure
Brochure
Company
Brochure
Brochure
Company
Brochure
Brochure
Upcoming Events
Current Month
No Events