©2023 Porton Advanced Solution Ltd. All Rights Reserved.
Porton Advanced Solutions is a subsidiary of Porton Pharma Solutions Ltd.
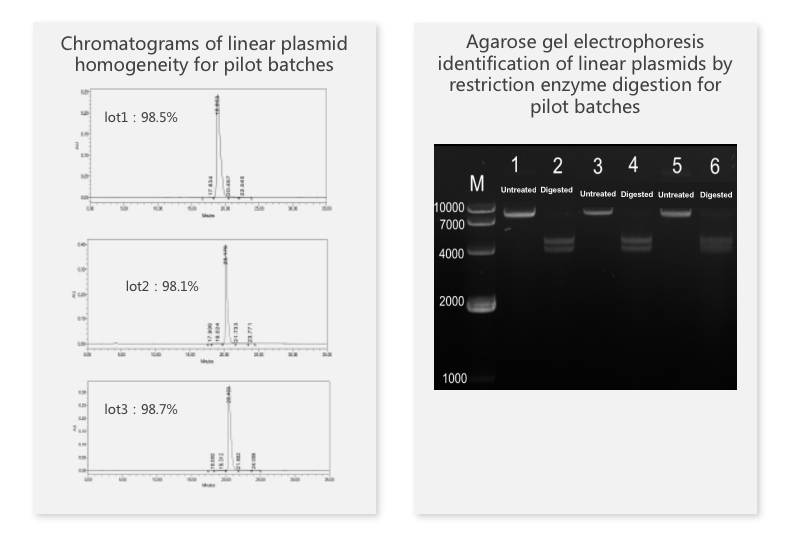



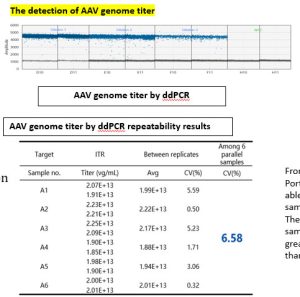
The repeatability study of selected samples at certain dilution levels showed great repeatability with a CV value of less than 10%.
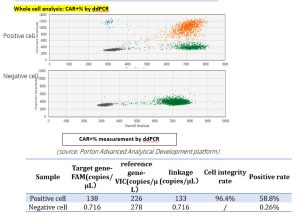
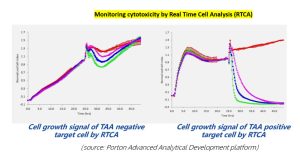
Case study
Assays

Case Study
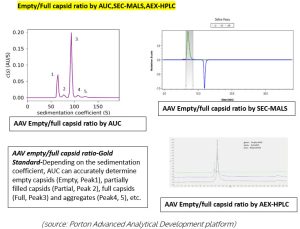
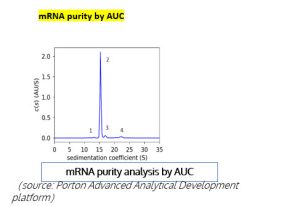
mRNA purity –AUC effectively distinguishes the different components in an mRNA sample, such as truncated mRNA (Peak 1), monomeric mRNA (Peak 2), long-chain mRNA, and mRNA aggregates (Peak 3& 4).
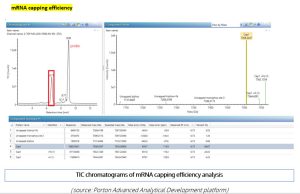
Figure 8
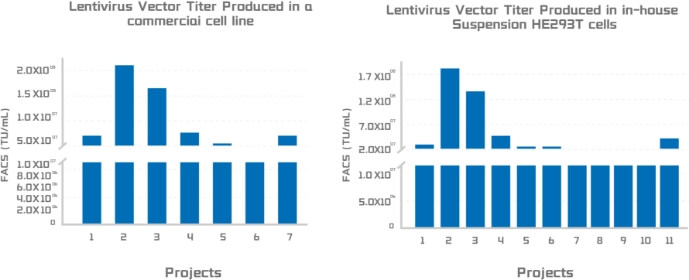
Lentivirus titer produced in two suspension cell production system:
A. Viral titer from a leading commercial packaging system. B. Viral titer from Porton Advanced’s PTLV-SMART packaging system.
Figure 4
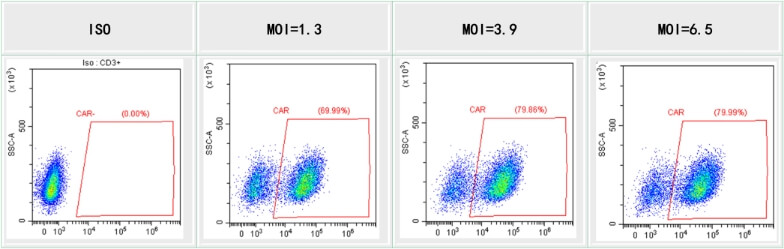
T cell transduction with lentivirus produced using our HEK293T system. T cells are infected with lentivirus at different MOI. CAR-T cell percentages were measured by FACS.
Figure 20
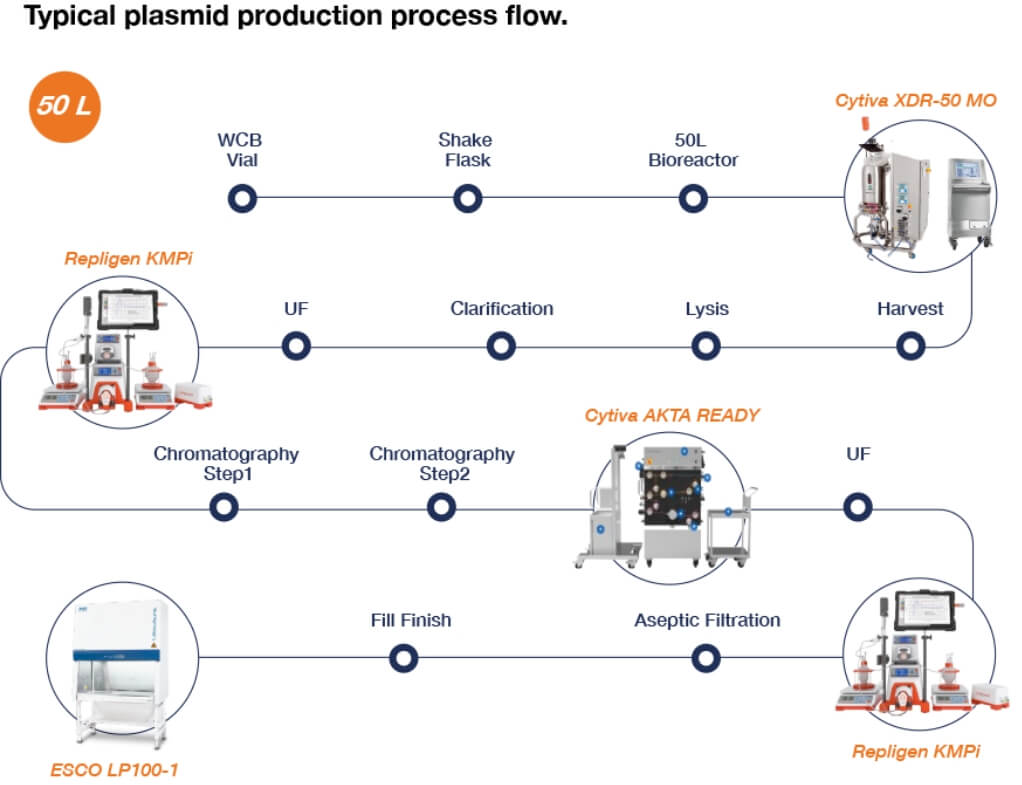
Figure 7
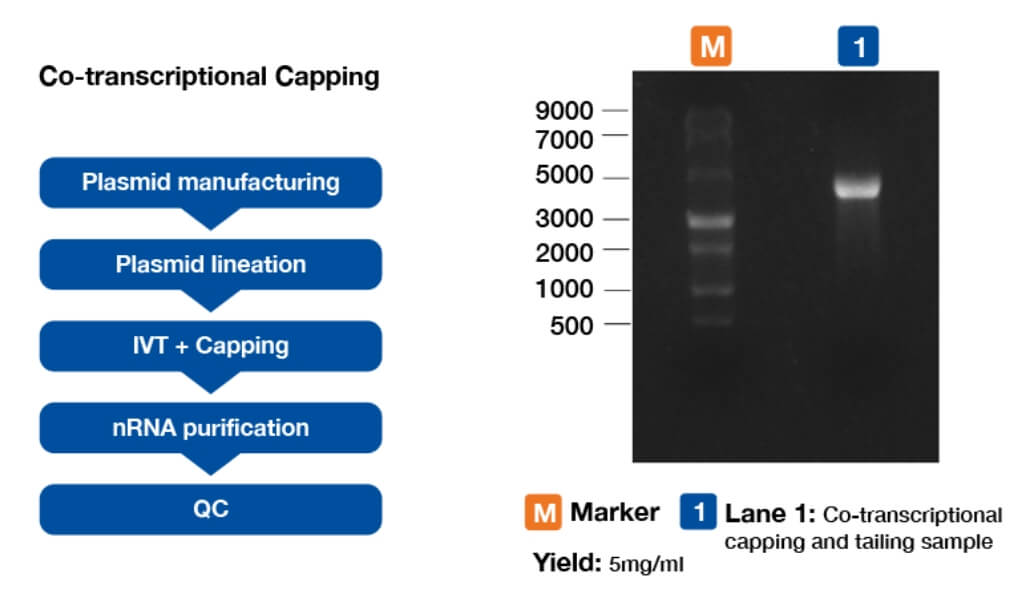
Figure 5
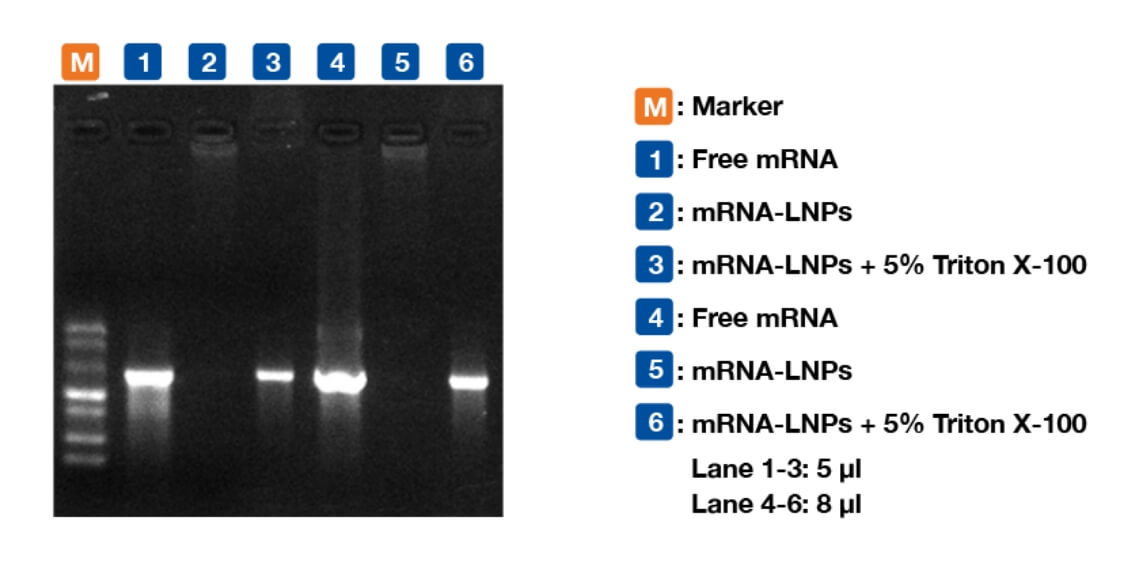
Figure 6

An AAV variant from our library screening showed that the virus was able to package a 6.1kb genome.
Figure 19
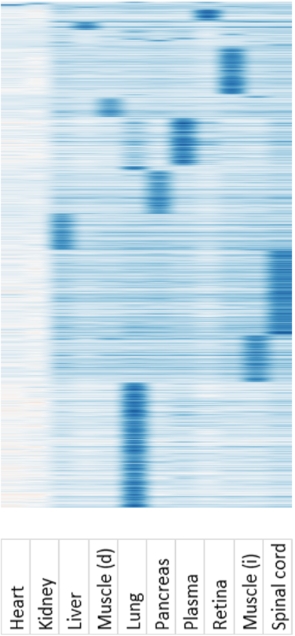
AAV variants enriched in different tissues during library screening. AAV libraries were prepared and injected into the hosts. After two weeks, tissues were harvested and viral genomes were isolated for PacBio sequencing. Viral genomes were clustered against different tissues.
Figure 18
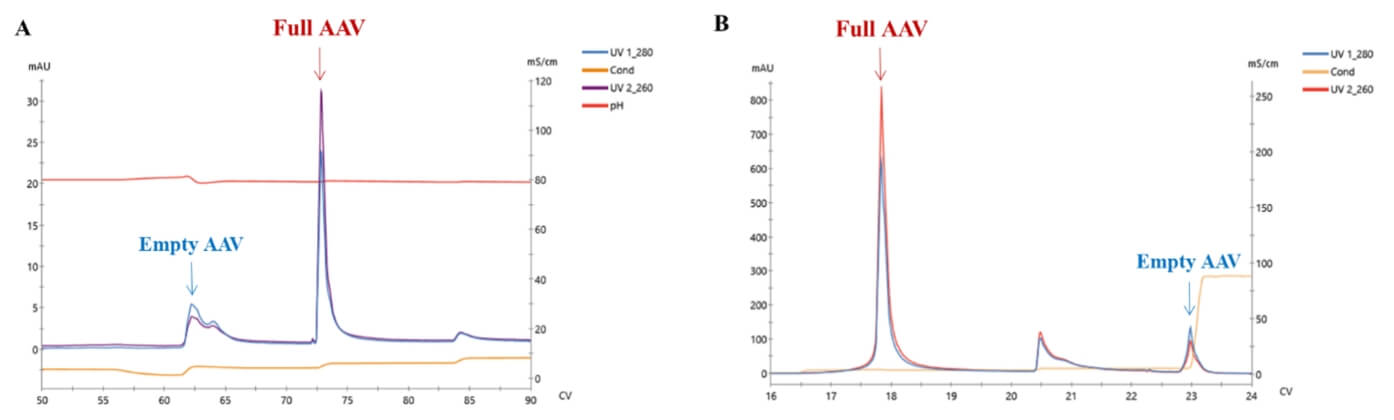
Separation of empty and full rAAV by anion chromatography. A: AEX result of rAAV5; B: AEX result of rAAV9
Figure 3
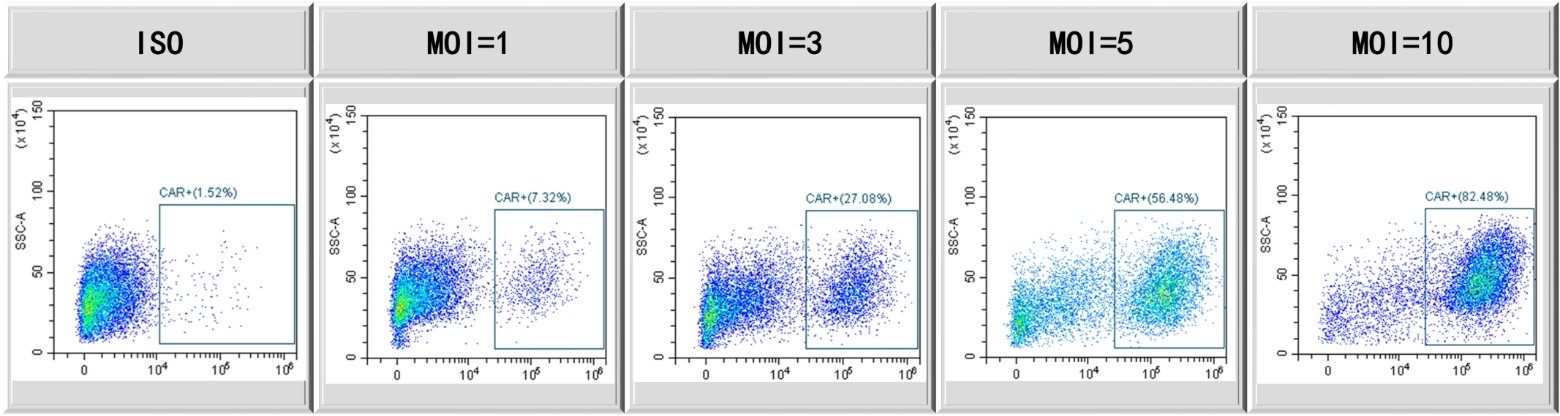
CAR expression with escalated MOI using PTLV-SMART platform. Activated T cells transduced with escalated MOI, and FACS employed to demonstrate CAR expression post-7 days culture in G-Rex.
Figure 1
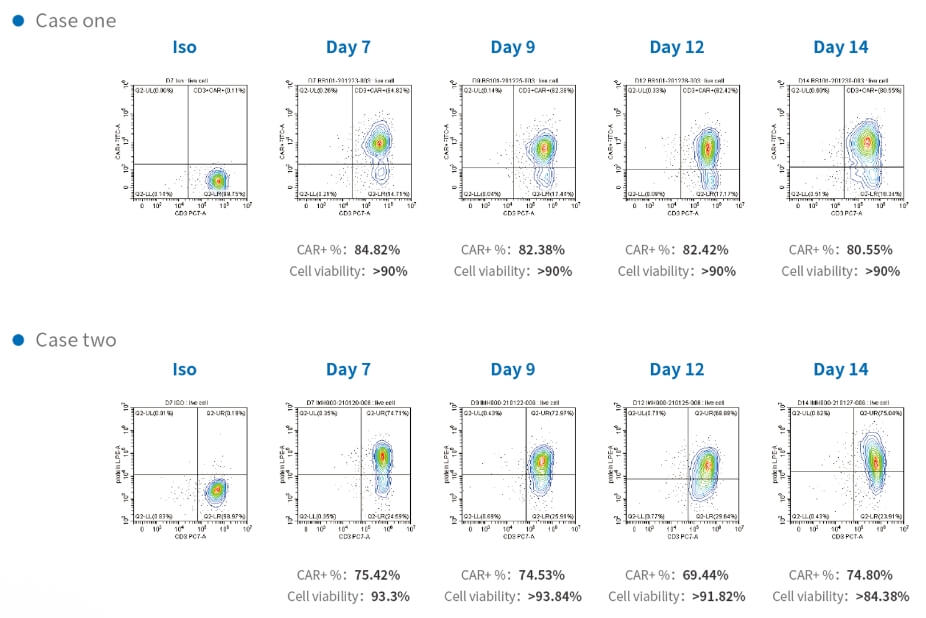
Time course of CAR transduction rates of CD3 T cells and cell viability during manufacturing in two cases. T-cells transduced with lentivirus analyzed by flow cytometry to detect CAR-positive or Protein L-positive cell ratio. T-cells expressed CAR stably in the period of CAR-T cell growth with high cell viability.
Figure 2