Analytical Development & Quality Control
Our analytical teams at Porton Advanced Solutions have enriched experience in method development, verification, validations in a wide range of Cell and Gene Therapy (CGT) products. By being partnered with more than 50 CGT projects, we have established our technical competencies in various testing platforms, including but not limited to CAR-T, viral vectors, bacterial vectors, and mRNA.
- Cost-effective quality control solution
- cGMP QC platforms in compliance with FDA, EMA, and NMPA filings
- Timely delivery of services and results
- Customized method development and validation services
Our analytical platform provides assays developed on advanced instruments and broad sample matrices to support the development and characterization of CGT. These state-of-the-art instruments include LC-MS, AUC, ddPCR, advanced microscopy systems, and cutting-edge flow cytometers, among others. These instruments precisely analyze various aspects of CGT, from the identification and quantification of specific molecules to the evaluation of therapeutic efficacy and safety. At Porton Advanced, we have developed more than 300 different assays and are providing standard testing services to our clients.
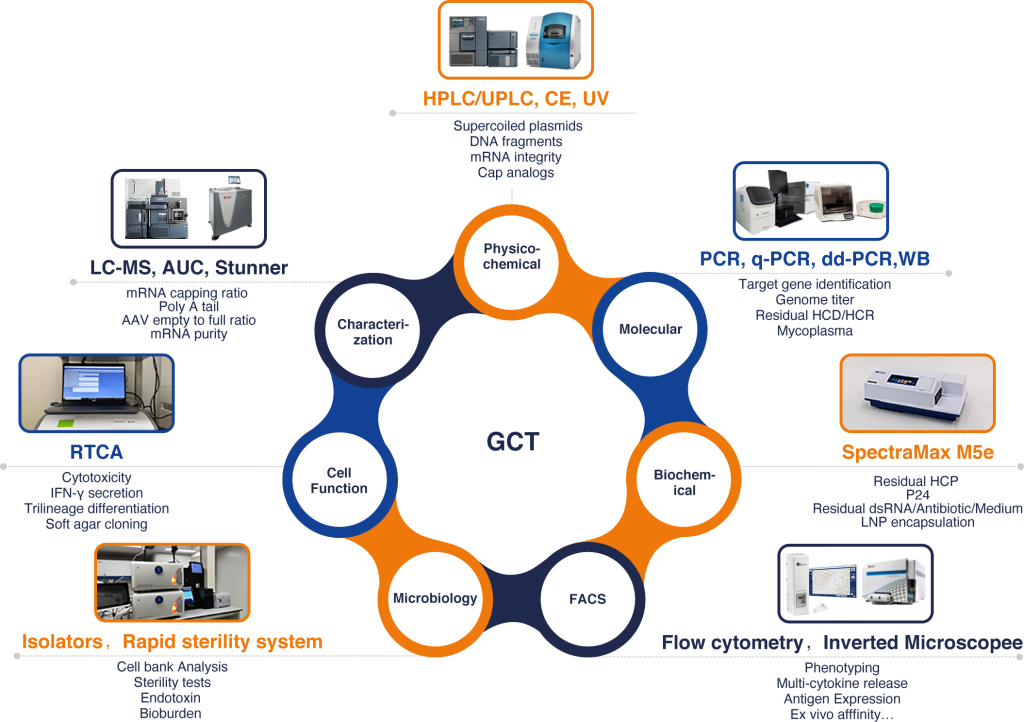

The seven functional platforms are based on the combination of advanced instruments and broad sample matrices empowering researchers and developers to gain comprehensive insights into the molecular and functional attributes of CGT. This optimizes production processes, identifies potential biomarkers, assesses product quality, and ensures the safety and efficacy of these innovative therapies. With an extensive array of analytical capabilities, Porton Advanced serves as a valuable resource for advancing the field of CGT, ultimately bringing novel treatments to patients in need.
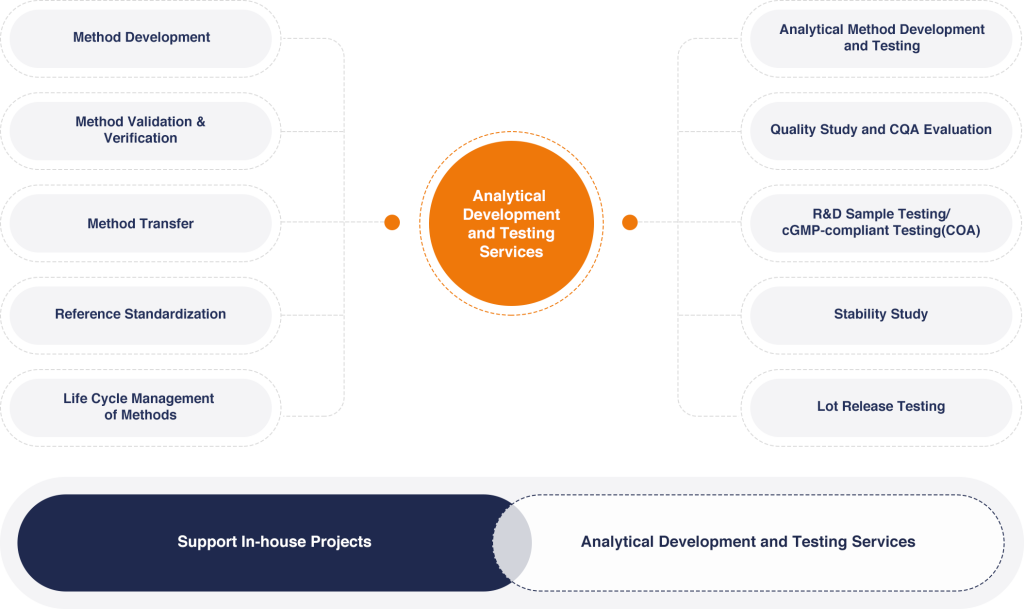
A wide range of analytical development and quality control services are being provided by our AD & QC team who possess strong expertise in process/product characterization, identification of critical quality attributes, specification and stability study design, testing services for CGT products, and c-GMP compliant lot release testing.
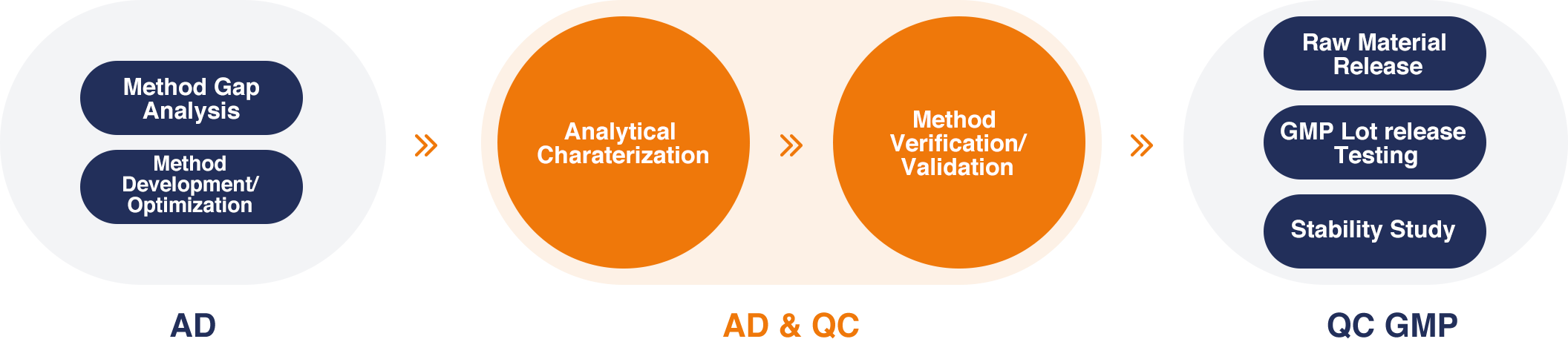
The Analytical Procedure Lifecycle Management
At Porton Advanced, we have built a robust system for analytical procedure life cycle management as we understand that the documentation from the AD & QC team accounts for over 70% of IND filings.
Therefore, we designed our process to provide integrated testing services from the preclinical to the commercial stage. To kick start a project, our AD team will do a method gap analysis depending on the sample matrix, and after considering the manufacturing process, they will initiate method development or method optimization dependent on the client transferring their internal method. The AD & QC team will then perform process characterization, and method verification/validation. During the GMP stage, the QC team will take ownership of performing lot release testing, raw material release, and stability study.