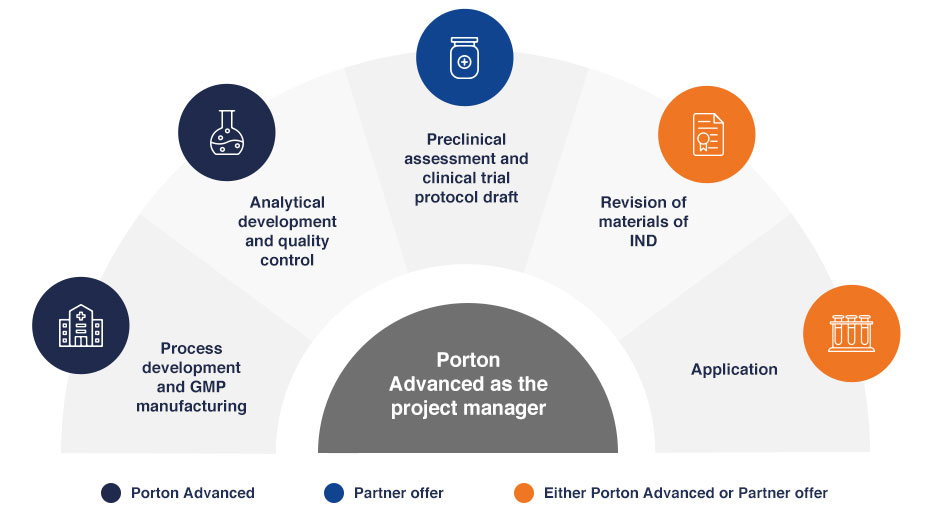
Porton Advanced created, and now exclusively offers, our own integrated IND-enabling study service. We lead IND-enabling studies in areas of process development and GMP manufacturing, pre-clinical services, clinical services, and regulatory affairs. We have successfully assisted our Chinese clients in obtaining 8 NMPA IND approvals, 4 FDA IND approvals, and 1 MedSafe IND approval.