
Investigator-Initiated Clinical Trial Services
Discover a world of possibilities
Discover a world of possibilities with Porton Advanced as we extend an invitation to explore our unparalleled suite of services, meticulously crafted for Cell Therapy Investigator-Initiated Clinical Trials (IIT), Investigational New Drug (IND) applications, even Phase 1/2 and NDA/BLA endeavors within China.

IIT areas we can assist with:
- Document preparation, submission, and approval from local authorities
- Connecting with local authorities and medical institutions
- Supporting the review of IIT study protocols
- Preparing documents as a CDMO partner to medical institutions for project approval and NHC registry review
- Finding reliable CRO and medical writing partners
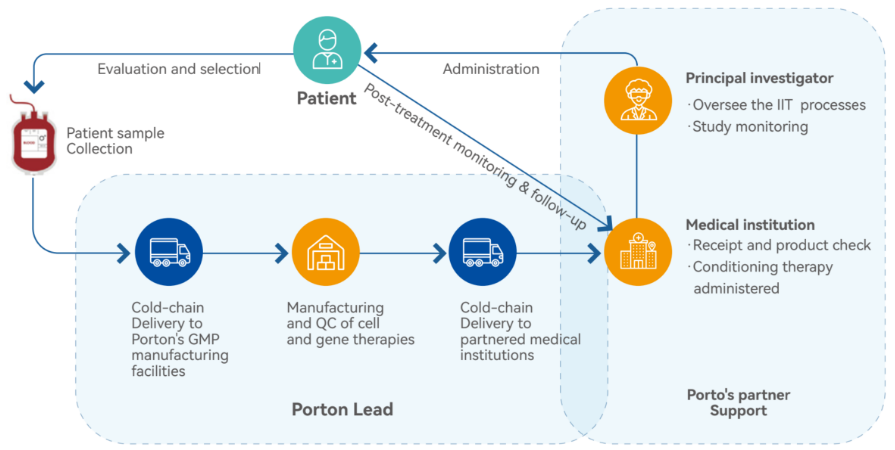
Conducting IIT
Proven Track Records: We have extensive CMC experience in CAR-T, TIL, CAR-NK/NK, MSC, iPSc, HSC, etc. and have completed 5 IIT projects (50+ batches with a 100% release success). Additionally, we have 4 ongoing Phase I/II projects (190+ batches scheduled within 2 years).
State-of-the-Art Facilities: Porton Advanced Solutions LLC is headquartered in NJ, USA and offers two state-of-the-art GMP facilities in Suzhou, China with a total 215,000 square feet. We have Cell Therapy-12 independent suites (2 for infectious donors), Viral Vectors- 10 production suites- (50-2000L), Plasmid – 2 independent suites (50L*2), Process Development & cGMP Production, Aseptic Fill and Finish, as well as Analytical development and Quality Control and more.
Strategic Partnerships: We have forged strategic alliances with key stakeholders in the industry such as principal investigators, hospitals, and regulatory agents, enabling us to leverage resources and networks to effectively accelerate your projects/clinical trials.
Time and Cost Savings: Opting to conduct Investigator-Initiated Trials (IIT) and seek Investigational New Drug (IND) approvals in China, while simultaneously applying for both NMPA and FDA approvals, offers substantial cost and time savings. With our proven track record, two of our customers succeeded in obtaining both NMPA and FDA IND approvals, and we presently have 2-3 customers in the process of applying for both NMPA and FDA IND approvals.
