VIRAL VECTORS – AAV
Technical team dedicated to AAV process development and cGMP production
We have a laboratory space of about 4,000 sq mt (43,000 sq ft), which can carry out early R&D, process development, and production of AAV, including upstream (USP) and downstream (DSP) process development, analytical development, and GMP production.
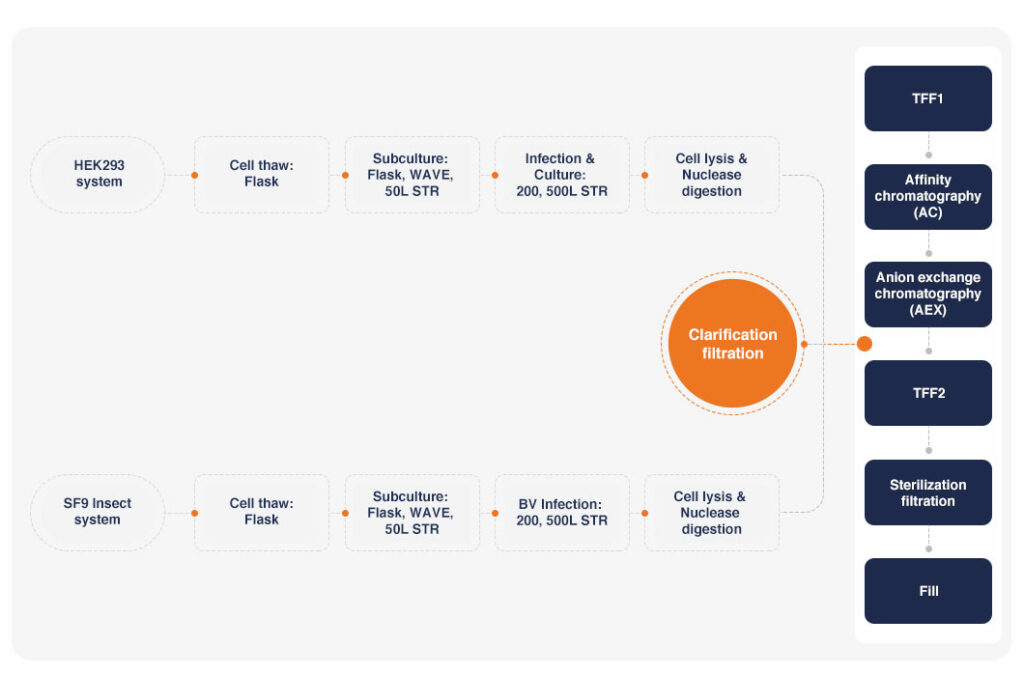
We provide end-to-end AAV gene therapeutic product development, including plasmid manufacturing throughout AAV upstream process development and downstream process development, and ultimately fill and finish. We offer both mammalian cell HEK293 and insect cell Sf9-based production systems, which provide flexibility to suit every client’s unique needs.
Our Services
Based on our professional capabilities, we can provide comprehensive AAV related services:
AAV PROCESS DEVELOPMENT
Upstream processing
Porton Advanced provides upstream process testing and development to assist customers in optimizing process parameters such as plasmid ratio, cell density, media, transfection reagents, pH, etc. to obtain the best upstream process parameters. We also provide upstream process development services for adherent and suspension production.
Our AAV manufacturing suites can reach a scale of 500L per batch (with a reserved production space of 2000L). Our expertise and collective experience, dedicated process development and analytical development teams, and state-of-the-art facilities and equipment make us a globally leading AAV CDMO.

Downstream processing
Our downstream process development teams can perform a variety of serotypes of AAV purification while supporting both column chromatography and ultracentrifugation. The optimization of the purification process involves various processes such as cell lysis, clarification, ultrafiltration concentration, affinity chromatography, ion exchange chromatography, sterilization and filtration, and formulation development, to obtain adeno-associated viruses with high purity, good activity, and low residue of impurities such as HCP/HCD, and to comprehensively consider production and process costs to confirm the optimal process parameters and purification route.
CGMP AAV PRODUCTION
From pre-IND to commercial production
Porton Advanced has a dedicated viral vector production team with decades of hands-on and management experience.
We have established SF9 and HEK293 system processes, with a maximum capacity of 80 batch/year. The cGMP facility has an independent air conditioning system, a single-use and fully enclosed aseptic production process, and a unidirectional flow of personnel and logistics to minimize the risk of contamination and cross-contamination. The whole production operation can be monitored by CCTV, which can be preserved for further traceability, and all the production equipment has completed CSV (Computerized Systems Validation). The facility design and quality system meet the requirements of cGMP production management systems in China, the US, and the EU, allowing us to provide CDMO services for global clients.
Support HEK293 and SF9-based AAV Production