Our Services
LV PLASMID PRODUCTION
Porton Advanced has completed numerous GMP productions of plasmids, including supercoiled plasmids for LVV, AAV, and linearized plasmids for mRNA.
Our facilities are equipped with level D cleanrooms for upstream fermentation, level C cleanrooms for downstream purification, and level B cleanrooms for the filling area.
Additionally, we offer ready-‐to use lentiviral helper/packaging plasmid products that have been registered with CBER FDA and also meet the requirements of both NMPA and EMA:
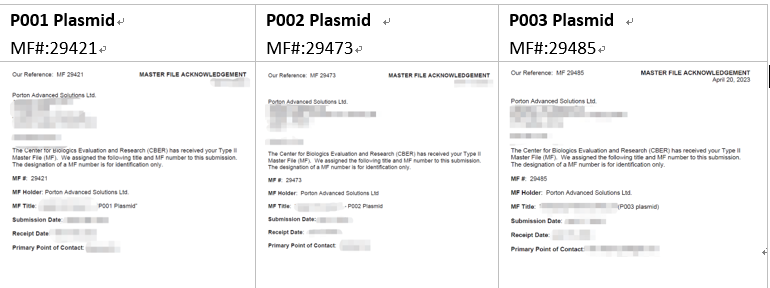
LV-SMART™ VSV-G (DMF #29485) – available in 1mg, 5mg and 10mg, please inquire within.
LV-SMART™ Rev (DMF #29473) – available in 1mg, 5mg and 10mg, please inquire within.
LV-SMART™ GagPol (DMF #29421) – available in 1mg, 5mg and 10mg, please inquire within.
Note:
1. For GMP grade, Minimum purchasing unit is 1 vial (5mg), for example, if you need 7mg P001 plasmid, you may buy 2 vials.
2. For Research Advanced (high purity plasmid) and GMPas (GMP-like), the minimum purchasing unit is 1 (1mg or 5mg) vial.
(P001, P002, & P003 plasmids have assisted multiple cell engineering projects).
Off-The-Shelf Product: Three Helper
Plasmids for LVV Production
Porton Advanced provides off-the-shelf helper plasmids accelerating the project development process from clinics. P001, P002, P003 plasmids have assisted multiple cell therapy projects to obtain China IND approvals and the corresponding DMFs have been successfully received by FDA.
These three helper plasmids have built master cell banks, as well as working cell banks. Porton Advanced can provide off-the-shelf products and CMC information, which ultimately shortens the project cycle. When using the helper plasmids for clinical and marketing applications of cell and gene therapy products in the US market, customers can directly cite the DMF number and refer to the LOA authorization letter provided by our team.
MASTER FILE ACKNOWLEGEMENT: