VIRAL VECTOR (LVV)
Proprietary LVV production system offering improved performance.
A lentivirus is a single-stranded RNA retrovirus that is widely used as a gene transfer tool in cell and gene therapies based on its ability to infect both dividing cells and differentiated non-dividing cells.
Traditionally, lentiviral vectors (LVVs) are manufactured using an adherent cell production platform with serum, but serum-free suspension cell systems have also been developed as an alternative. At Porton Advanced, we have developed a proprietary suspension cell line HEK293TG2S through clonal screening, suspension adaptation, and viral vector production technologies.
Based on the concept of quality and design from advanced technologies, we have developed a HEK293T Lentiviral Vector production platform (LV-SMART™), which has been successfully used in more than a dozen client projects and has exceled in multiple CAR-T/TCR-T tests.
Our Services
Porton Advanced offers the following LVV services:
Porton Advanced also provides other commercial LVV suspension production platforms, as per our clients’ request. We have successfully supported several of our clients using LV-SMART™ and commercial LVV systems in filing Investigational New Drug Applications (INDs).

LVV PROCESS DEVELOPMENT
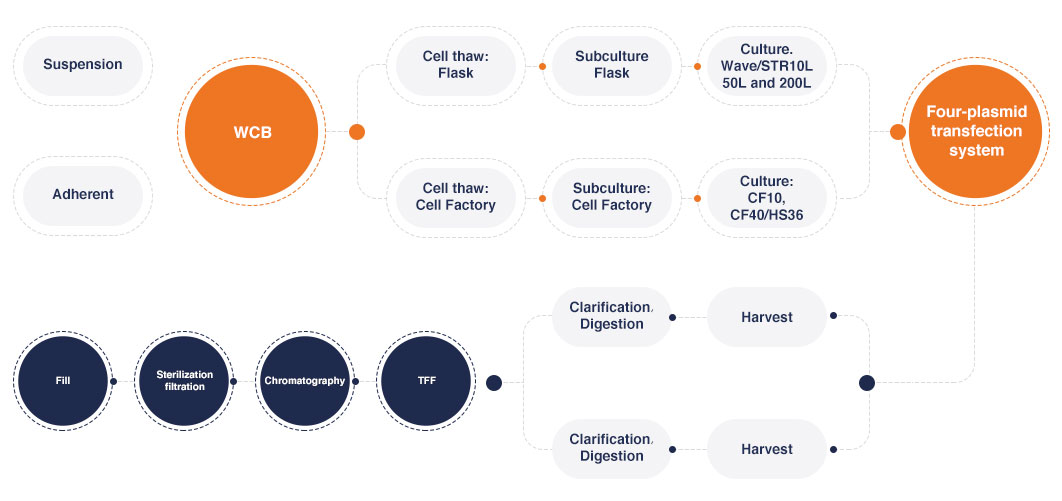
Upstream processing
To obtain Lentiviral Vectors with high titer, low impurities, and low production cost, the experienced technical team at Porton Advanced conducts in-depth and comprehensive research on the upstream processing of LVVs. This includes screening and exploration of factors such as basic culture medium, cell density, ratio of helper plasmids to GOI plasmids, quality dosage, transfection reagents, additives, and virus harvest time to determine the optimal process route, process parameter range, and most suitable raw materials. Currently, we have conducted feasibility tests for over 100 different CAR/TCR sequences and provided services for multiple CMC projects. The highest titer was above 1E+08 TU/mL.

Downstream processing
In order to obtain LVVs with high purity and controlled production costs, the experienced technical team of Porton Advanced has conducted in-depth and comprehensive research on the development of virus purification process, including the screening and exploration of virus harvest solution clarification, enzyme digestion, column chromatography, UF/DF, sterilization filtration and formulation. To determine the optimal process route, process parameter range and the most suitable raw materials. LVV produced based on the LV-SMART™ has the advantages of good virus activity, low impurity residues, low production cost, simplified production process and high virus recovery rate.
CGMP LVV PRODUCTION
From pre-IND to commercial production
Porton Advanced has a dedicated viral vector production team with decades of hands-on and management experience.
We have established robust production processes for adhesion and suspension production with a maximum of 200L per batch and 100 batches per year. The cGMP facility has an independent air conditioning system, a single-use and fully enclosed aseptic production process, and a unidirectional flow of personnel and logistics to minimize the risk of contamination and cross-contamination. The whole production operation can be monitored by CCTV, which can be preserved for further traceability, and all the production equipment has completed CSV (Computerized Systems Validation). The facility design and quality system meet the requirements of cGMP production management systems in China, the US, and the EU, allowing us to provide CDMO services for global clients.
Support Suspension and Adherent cGMP LVV Production
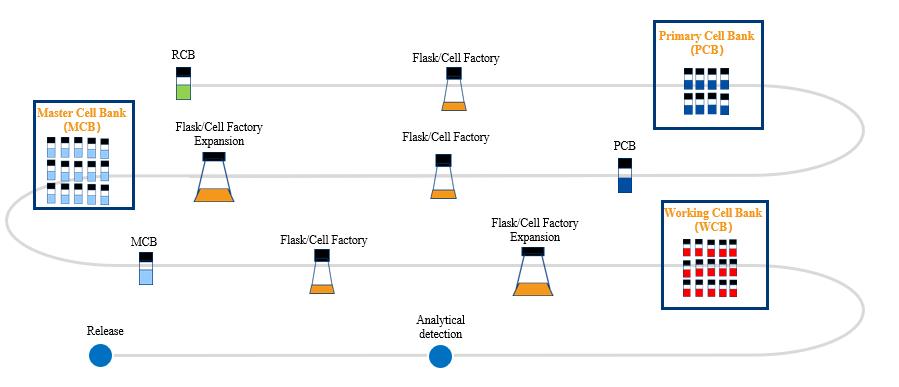
Cell Banking Service
Porton Advanced provides three-tiered cell banking service based on adherent and suspension cell lines that can be used for eCTD publishing and submissions to the FDA and NMPA. Cell lines include HEK293/T/F,HeLA, etc. Our technical team will establish a three-tiered cell bank in a cGMP environment and perform programmed cooling and cryopreservation to maximize cell viability. We have successfully delivered several projects, one of which has led into commerical stage.