High quality plasmids at reduced cost
As a carrier of target genes, plasmids have a significant impact on the quality, safety, and effectiveness of the gene and cell therapy final products.
Our teams have years of proven experience in plasmid manufacturing, including construction optimization, colony selection, and GMP manufacturing process and analytical development. We understand the importance of lowering the production cost without compromising the quality, safety, and effectiveness of the final CGT products.
Our Services
We customize process development procedures based on final applications:


Plasmid Process Development
Porton Advanced’s process development team has extensive experience in establishing process platforms for different applications of plasmid:

CGMP PLASMID PRODUCTION
Supporting stainless steel and
single-use production
In order to meet different customer needs, Porton Advanced has established two production lines including one disposable and one stainless steel perfusion production lines with a capacity of 80 batches per year. Currently, we have delivered dozens of GMP production of plasmids, including supercoiled plasmids for LVV, AAV and linearized plasmid for mRNA, and more. Such products meet a high requirement within the industry. In addition to that, the master files for three helper plasmids used for LVV products have been received by CBER.
Our plasmid production facility is compliant with biosafety level 2 (BSL-2) protocols. Different production areas are equipped with independent air conditioning systems, diversified
cleanroom levels, pressure differences, temperature, and humidity control. The upstream fermentation area is a cleanroom level D, whereas the downstream purification area is a cleanroom level C, and the filling area is a cleanroom level B. All the cleanrooms can effectively meet the requirements of plasmid GMP production.
Microbial Banking Service
Provide banking service (MCB/WCB), which meets the declaration requirements of NMPA, FDA and EMA.
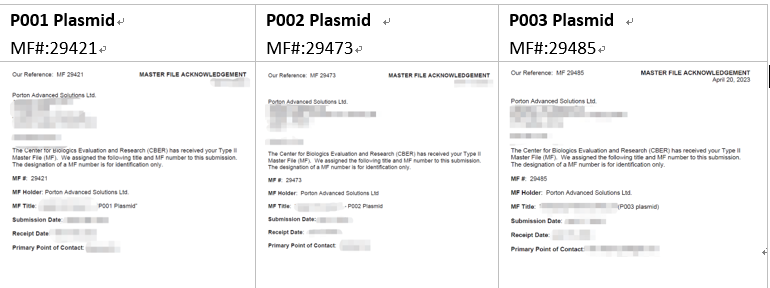
Off-The-Shelf Product: Three Helper Plasmids for LVV Production
Porton Advanced provides off-the-shelf helper plasmids, that meet the requirements of NMPA and EMA, accelerating the project development process from clinics. P001, P002, P003 plasmids have assisted multiple cell therapy projects to obtain China IND approvals and the corresponding DMFs have been successfully received by FDA.
These three helper plasmids have built master cell banks, as well as working cell banks. Porton Advanced can provide off-the-shelf products and CMC information, which ultimately shortens the project cycle. When using the helper plasmids for clinical and marketing applications of cell and gene therapy products in the US market, customers can directly cite the DMF number and refer to the LOA authorization letter provided by our team.
The MASTER FILE ACKNOWLEGEMENT
is as follows:
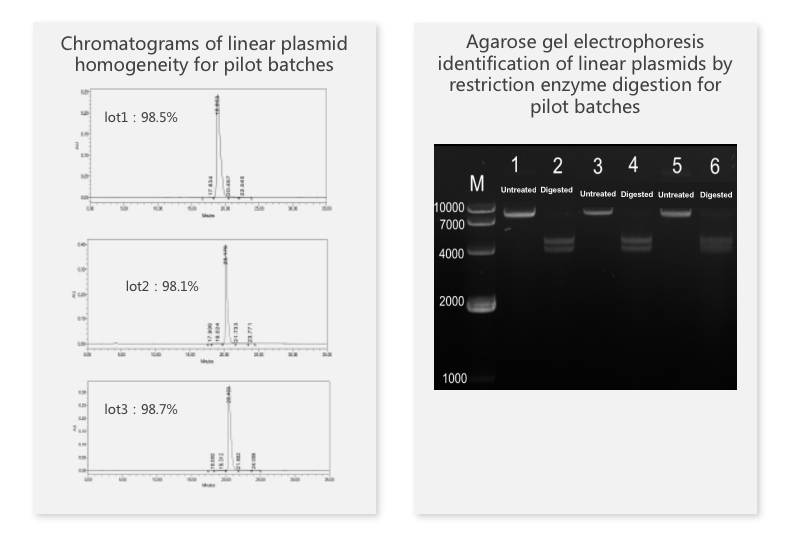
Linearized plasmid case study
Supercoiled and Linearized
Plasmid Production
Porton Advanced uses a high density fermentation, in-line lysis process to produce high-quality plasmids that meet customer needs. At the same time, we can provide high-quality linearized plasmids for mRNA production. The quality of linearized plasmids directly affects the IVT effect of mRNA. We have successfully helped our customers obtain IND approvals in the United States and New Zealand.
Production Process Workflow

