mRNA
High quality mRNA at high yields
mRNA is a promising and rapidly growing new type of advanced medicine with quick production speed, the ability to encode varied proteins, an excellent safety and efficacy profile, and flexibility in modality and formulation. We are able to provide end-to-end CRO (click here for mRNA CRO service) and CDMO services. Based on the well-established and high-efficient mRNA platform, we continue to acceralate your products from clinics to beyond.
At Porton Advanced, we have established an expert mRNA manufacturing process, including USP mRNA production (IVT, enzymatic/co-transcriptional capping poly(A) tailing, etc. with yield up to 10mg/mL), DSP mRNA purification (affinity chromatography, ion-exchange polishing, TFF, etc.) and lipid nanoparticle (LNP) delivery formulation. The validated process can greatly reduce the project timeline and deliver results with robust quality and high efficiency.
Our Services
We are a trusted partner to our clients, providing end-to-end services from plasmid process development to GMP mRNA manufacturing:
mRNA Process Development


mRNA Case studies
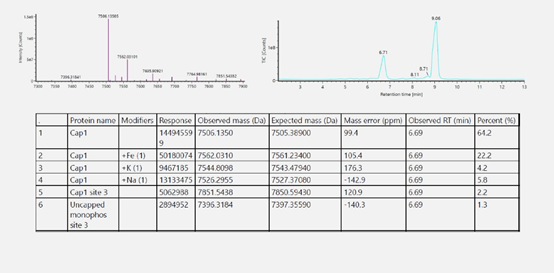
Porton Advanced’s Analytical platform utilized the CGE method for assessing mRNA integrity. Results showed a high level of mRNA intactness, meeting the process development standa
 (source: Porton Advanced’s Analytical Development Platform)
(source: Porton Advanced’s Analytical Development Platform)
Porton Advanced’s Analytical platform utilized the CGE method for assessing mRNA integrity. Results showed a high level of mRNA intactness, meeting the process development standard.

(source: Porton Advanced’s Analytical Development Platform
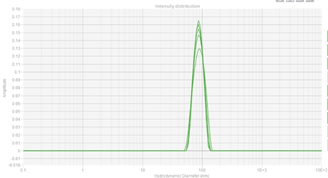
Porton Advanced’s analytical platform showed the results on particle size distribution of LNP sample by SEC-MALS to be less than 0.1, proving the stable process delivered by the process development team.ing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
(source: Porton Advanced’s Analytical Development Platform)
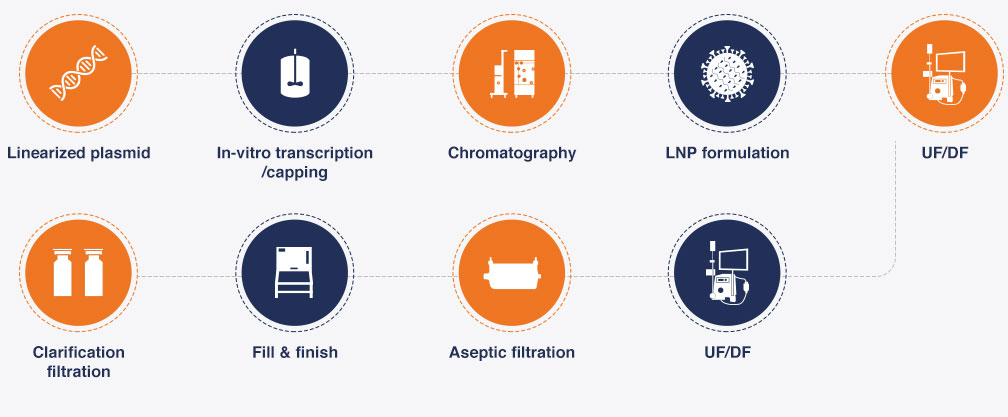
cGMP mRNA PRODUCTION
Production work flow